Artificial ovary could allow women to become mothers after cancer treatment without risk, say scientists

Pregnancy after 40? Yes indeed!
September 12, 2019
Goldman Sachs’s egg-freezing scheme is a pathway to a hellish dystopia
November 8, 2019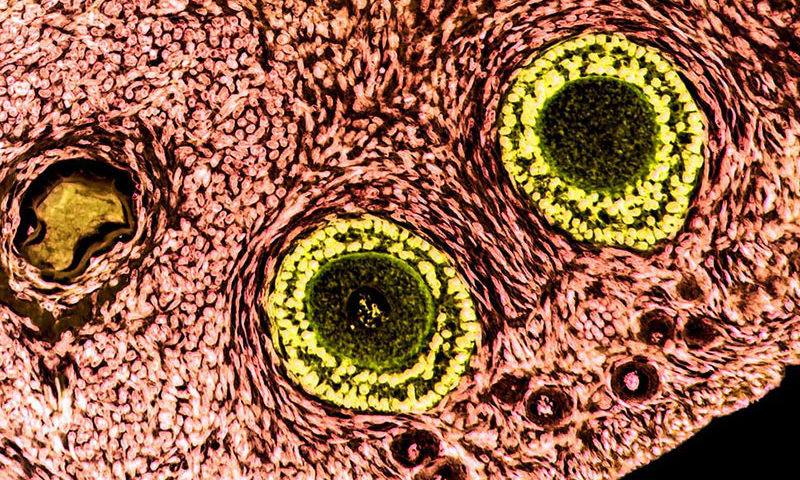
An artificial ovary that can grow immature eggs into a fertilizable cell fit for implantation could one day let women become mothers after fertility-destroying cancer treatment, scientists have said. Researchers from one of the leading fertility centers in Europe, Rigshospitalet in Copenhagen, say they have demonstrated the world’s first working “bio-engineered” human ovary in early animal trials.
Currently women will have eggs frozen before chemotherapy or radiotherapy, which can destroy the viability of the cells.
Prepubertal girls or women who need urgent treatment before they produce eggs, rely on ovarian tissue, which contains thousands of immature eggs in fluid-filled sacs called follicles, to be preserved instead with the aim of transplanting it after treatment. However, if there is a risk of any cancerous cells remaining in preserved tissue, re-implantation could be deemed too risky.
The Danish technique offers a way to avoid this, and potentially improve on IVF and other techniques as more potential eggs could be preserved and would develop naturally if they can be re-implanted.
The team used a chemical process to strip the ovarian tissues’ cells of DNA and other features which could contain the faulty instructions for cancer cells’ unconstrained growth.
They then implanted immature egg cells into this empty ovarian “scaffold”.
The team showed that the immature eggs and tissue scaffold could reintegrate and survive in this scaffold, and it could then be grafted into a living host – in this case a mouse.
In theory the eggs would begin to mature and release each month in line with the hormone cues of the menstrual cycle.
“This is the first time that isolated human follicles have survived in a decellularised human scaffold and, as a proof-of-concept, it could offer a new strategy in fertility preservation without risk of malignant cell re-occurrence,” said Dr Susanne Pors, who led the Rigshospitalet team.
Dr Pors added that the risk of cancer returning from re-implanted ovarian tissue is “real”, though her team and others have found the chances are low.
It comes after a UK team were the first to take immature cells from follicle to mature eggs outside of the body, but without an ovarian scaffold that could allow follicles to be re-implanted and develop naturally.
The latest study has yet to be published in a peer-reviewed journal and is presented at the European Society of Human Reproduction and Embryology (ESHRE) annual meeting in Barcelona on Monday.
But independent doctors said it was “groundbreaking” work, although the process now needs to be refined and shown to be safe in humans – which will take several years.
“This is an extremely important advance in the field of fertility preservation,” said Professor Adam Balen, professor of reproductive medicine and surgery at Seacroft Hospital, Leeds.
“The ability to successfully create a ‘new ovary’, by removing any tissue that might potentially reintroduce the cancer and fashioning a scaffold on which to grow the egg-containing follicles, allows the re-implantation of a ‘safe’ ovary, with the potential to successfully restore fertility.”
Dr Stuart Lavery, a consultant gynecologist at Hammersmith Hospital, said: “If this is shown to be effective, it offers huge advantages over IVF and egg freezing.
“Because potentially these small pieces of tissue will have thousands of eggs and clearly, if it does work, there’s the advantage of then getting pregnant the old-fashioned way.
“We are some years away from that, and so IVF and egg-freezing is here now and will be with us for several years, but if this works it has dramatic potential.”
Source: www.independent.co.uk